Temperature Monitoring Ensures Quality Assurance & Control
 Refrigerators and freezers are integral to the daily operations of most clinical laboratories, particularly those engaging in blood supply management, therapeutic blood product research, and blood-related disease management and research. At Massachusetts General Hospital, the diabetes laboratory, which performs all outpatient HbA1c testing for the hospital’s clinics, is entrusted with thousands of blood, serum, and urine specimens for numerous past, present, and ongoing clinical studies pertaining to diabetes. Although the performance of refrigeration equipment often is an afterthought to more dynamic lab technologies and operations, a temperature monitoring system is critical to the integrity of our patient samples and information. After collecting specimens for years, losing them due to improper storage practices, poor temperature stability, or malfunctioning equipment is unthinkable.
Refrigerators and freezers are integral to the daily operations of most clinical laboratories, particularly those engaging in blood supply management, therapeutic blood product research, and blood-related disease management and research. At Massachusetts General Hospital, the diabetes laboratory, which performs all outpatient HbA1c testing for the hospital’s clinics, is entrusted with thousands of blood, serum, and urine specimens for numerous past, present, and ongoing clinical studies pertaining to diabetes. Although the performance of refrigeration equipment often is an afterthought to more dynamic lab technologies and operations, a temperature monitoring system is critical to the integrity of our patient samples and information. After collecting specimens for years, losing them due to improper storage practices, poor temperature stability, or malfunctioning equipment is unthinkable.
Depending on the intended use of a specimen, the lab will process patient samples first and then freeze them for later use, or freeze samples first and process them later. Regardless, even minimal temperature fluctuations can affect sample integrity and, thereby, study outcomes. Thus, robust and secure medical-grade refrigeration systems are integral to the quality of our work.
FIGURE 1
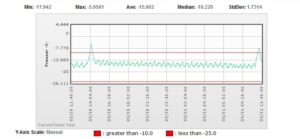
Sensor Chart
The graph reveals 1 week of temperature monitoring, with each green point representing a temperature reading. The red line is the lab’s high limit set point; if the temperature rises above that line, alarm notifications are sent to a list of specified contacts.
Data Capture for QA and QC
Acquiring medical-grade refrigerators and freezers specific to their intended use is just the first part of an overall program; system management policies and procedures {P&Ps) that include quality assurance (QA) and quality control (QC) measures also are required to maintain and monitor temperature control equipment. When choosing from among the many types of temperature monitoring systems available, consider such basic factors as hard-wired versus wireless data transmission; single-site or multiple-site monitoring requirements; alert and data capture mechanisms; and power and data storage redundancy. The diabetes lab and a number of other areas in the hospital employ a wireless, internet-based system that provides 24/7 monitoring and status alerting for all temperature control devices. Because device sensitivity is integral to the effectiveness of temperature-controlled equipment, P&Ps must address both routine and unique use parameters. A refrigerator or freezer may be opened and closed many times during the course of a day. The frequency and duration of device access without causing harm to specific specimens is equipment-dependent; some units are more sensitive than others.
Therefore, P&Ps should direct users to anticipate what will be placed in, or removed from, a unit before opening the door. In addition, it is important to monitor how quickly the temperature increases and returns to its set point each time the device is accessed. For some systems, it is better to open the equipment, perform some actions quickly, and then shut the door for a few minutes before proceeding to minimize temperature fluctuation of the contents. Although fluctuations may not affect specimens instantly, both clinical and regulatory requirements exist for temperature maintenance, and P&Ps must reflect and reinforce these.
QA for Lab Refrigeration Equipment
As with any new information system, it is important to understand fully the information the chosen temperature monitoring system provides, how to access it, and how to optimize its use. Our online monitoring system pulls trending temperature data from wireless monitors inserted inside each refrigerator and freezer in the lab. With acceptable temperature ranges and alert settings established, the team sought to design a redundancy so that no alarm goes unattended. This is where the system leaves off, and a well-crafted QA plan takes over.
To develop a temperature monitoring QA plan, a team of relevant practitioners was established, including the medical technologist, lab manager, lab director, and technical consultant. The resulting plan mapped the alert contact tree, designated the method of contact, and detailed the response to each alert. In our system, primary and secondary contact is assigned to each piece of monitored equipment. In the event of a device alert, the primary contact is notified first via telephone or email (users can choose). Sequential alarming is an option in the telephone system. When this feature is employed, the system notifies each person on the contact list in order. A user takes responsibility for the alarm by dialing 5, thereby halting the system from alerting additional contacts. Alternatively, users can program the system to reach out to all contacts simultaneously, in which case the contacts must decide who will respond.
The monitoring system will alert you any time a problem exists, and responders can log into the system from any Web-enabled device to assess the situation. In addition to alarms caused by temperature excursions, other common alerts include loss of Internet connectivity, internal network issues, device power outages, device power failures, and extended door ajar. If the temperature within a specific device goes out of acceptable range and the system cannot identify a reason, the primary contact must investigate, even if that means visiting the lab during off hours. One of the system’s advantages is that users can enter case comments through the system’s Web portal. For example, if a primary responder finds a malfunctioning wireless transmitter, this can be noted to prompt an engineering response, as well as facilitate tracking of equipment life cycles for timely replacement.
QC for Lab Refrigeration
If specific devices go out of range or the power falters without explanation, engineering maintenance or replacement must be considered. The monitoring system software tracks temperature shifts graphically, and data are reviewed weekly to ensure the ongoing integrity of the equipment (see FIGURE 1).
Prior to the installation of the wireless monitoring system, technicians used paper log sheets to manually record the device temperatures once per day. This system was particularly vulnerable to error due to the long periods between reporting and the lack of accountability. To improve on this, chart recorders were placed on the front of each refrigeration device. While the recorders illustrated temperature fluctuations tied to specific times, they did not provide immediate alerting. Now, wireless monitoring devices provide real-time information that is available to any authorized user over a secure Web site for all refrigeration equipment. Users no longer wonder if the temperature is a few degrees warmer due to an issue that occurred the previous night or weekend.
Trends Reveal Failure Points
If a freezer is set to -80°F but reads a couple of degrees warmer without returning to the set temperature, it probably means that the interior needs to be defrosted and cleaned. Ice can accumulate on the internal coils reducing airflow and the ability to provide cooling. Frost can also damage samples, and a build-up of ice can impede the door from closing and locking correctly. The goal is to keep all freezer units frost-free through regular cleaning; however, prudence is required, as over-cleaning introduces other problems. The process requires moving the specimens to a second freezer, which jeopardizes both the specimens being moved and those already in the second unit.
In addition to impromptu cleanings, we conduct a thorough cleaning and defrosting of the entire system bi-annually. Although this requires careful planning and sufficient time to relocate the specimens and unplug the equipment, ignoring maintenance places the equipment at greater risk of malfunction and the specimens at greater risk of loss.
Conclusion
The value of reliable and purpose-designed refrigeration systems cannot be overstated for clinical and research laboratories. Once a laboratory determines which system best suits its workload, the focus must shift to maintaining the integrity of the equipment and the specimens housed therein. In the research setting, months and possibly years of work can be lost to equipment malfunction, misuse, or improper monitoring or maintenance. QA and QC plans help ensure ongoing system integrity and can forecast potential failure points and facilitate proper response mechanisms. An online monitoring system enables users to track changes and trend data, which facilitates proper maintenance and, in turn, maximizes device longevity. Given the value of blood products and specimens, laboratory workers need to have confidence in the equipment that protects those specimens around the clock. The best way to do this is with a medical-grade, automated monitoring system.
About The Author
This Application Note has been adapted from an article written by Amanda Griffin, MT(ASCP). Griffin is the laboratory manager at the Massachusetts General Hospital Diabetes Laboratory, where she has worked for 16 years. In the past, she helped open the hemoglobin A1c lab in the Massachusetts General Hospital Diabetes Clinic.
For more information on our temperature monitoring systems, or to find the ideal solution for your application-specific needs, contact a CAS DataLogger Application Specialist at (800) 956-4437 or request more information.

